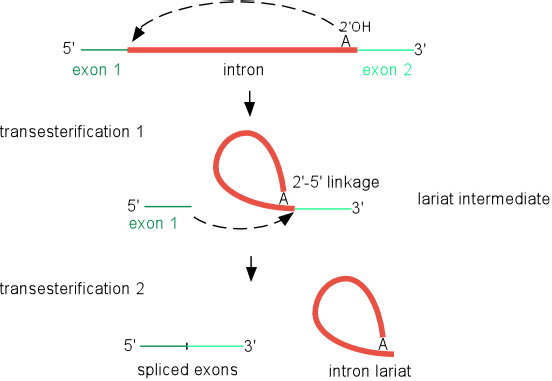
Splicing of group II introns
Self-splicing reaction
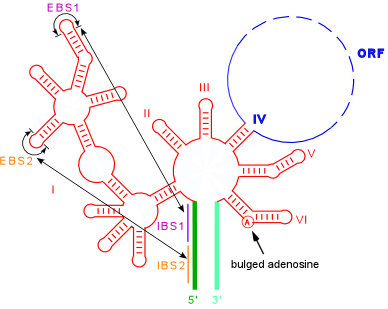
Splicing occurs through two sequential transesterification reactions. In the first step, a bulged adenosine in domain 6 attacks the 5' splice site. This results in cleavage of the 5' exon and the formation of a lariat intermediate product. The 5' exon remains held in place by the ribozyme by the IBS1-EBS1 and IBS2-EBS2 pairings. In the second step, the 5' and 3' exons are ligated together and the intron is released as a lariat. To the detail drawn, this reaction is identical to the splicing of nuclear pre-mRNA introns, supporting the idea that group II introns were ancestors of spliceosomal introns.
Secondary structure showing the IBS-EBS pairings and bulged A utilized during the splicing reaction
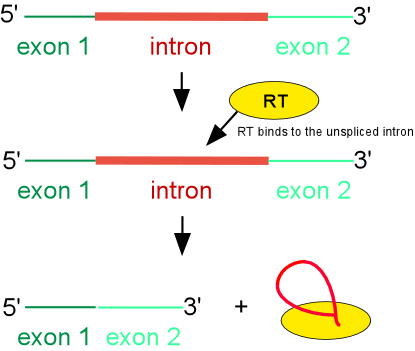
Maturase-assisted splicing reaction
Although some group II introns self-splice in vitro, splicing in vivo requires the assistance of protein. The most important splicing factor is the intron-encoded RT. For the well studied Ll.LtrB intron of Lactococcus lactis, the IEP binds to unspliced intron RNA at a high affinity binding site in domain 4A and makes secondary contacts in domains 1, 2 and 6. Together, the protein-RNA interactions result in conformational changes, or structural stabilization, that result in self-splicing. After splicing, the IEP remains tightly bound to spliced intron, and this ribonucleicprotein (RNP) particle is the active moiety in subsequent mobility reactions.