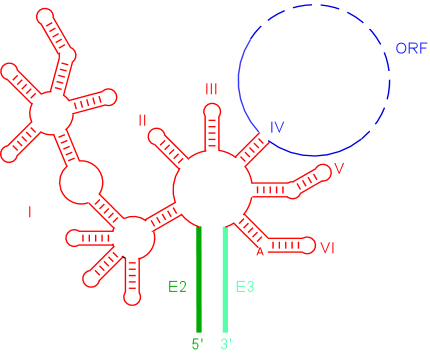
Structure of group II introns
Group II introns fold into a conserved secondary structure consisting of six domains arranged around a central wheel. Domain 1 is the largest and varies substantially in sequence and secondary structure between the various subclasses of group II introns. Domain 5 is highly conserved in sequence and together with domain 1 forms the minimal catalytic core of the intron. Domain 3 acts to enhance the catalytic rate, while domain 4 is not conserved in sequence but encodes the IEP. Domain 6 contains a bulged adenosine residue, whose 2'-OH is the nucleophile that initiates the splicing reaction.
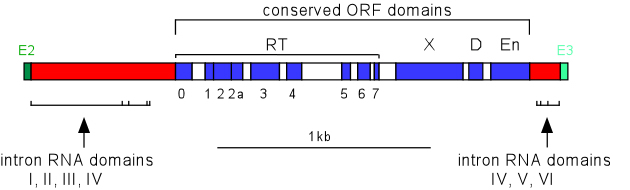
For introns that encode open reading frames (ORFs), the start codon is typically found within the loop of domain 4. The ORF contains the seven domains typical of RTs, as well as domain X, domain D and the En domain. Domain X is analogous to the thumb domain of other polymerases, and is associated with the maturase (splicing) activity of the protein. The D domain is a DNA binding region, while the En domain contributes an endonuclease activity utilized in the mobility reaction. Some group II introns, including IIC introns (bacterial class C), lack the En domain and rely on DNA replication forks to provide the primer during the mobility reaction.